
Advances and challenges in improvement of the electrochemical
It has been recognized as one of the most effective and practical options to development the corresponding electric energy storage technologies, such as electrochemical

(PDF) Multiphysics Engineered Next-Generation
This report explores advancements in lead-acid battery technology, focusing on innovations that enhance their application in electric vehicles (EVs) and energy storage systems.

Advances and challenges in improvement of the electrochemical
As shown in Fig. 3a, the ideal battery as an energy storage system should have the characteristics of high specific energy, high power density and good fast charging

Lead-Acid Batteries: The Cornerstone of Energy Storage
Lead-acid batteries have their origins in the 1850s, when the first useful lead-acid cell was created by French scientist Gaston Planté. Planté''s concept used lead plates submerged in an

Energy Storage with Lead–Acid Batteries
As the rechargeable battery system with the longest history, lead–acid has been under consideration for large-scale stationary energy storage for some considerable time but

Electrochemical Energy Storage
Open access peer-reviewed chapter2.1. Lead acid battery Lead acid battery when compared to another electrochemical source has many advantages. It is low price and availability of lead, good reliability,

Advanced Lead–Acid Batteries and the Development of Grid
This paper discusses new developments in lead-acid battery chemistry and the importance of the system approach for implementation of battery energy storage for renewable

Lead-Acid Battery Technologies: Fundamentals,
Lead-Acid Battery Technologies: Fundamentals, Materials, and Applications (Electrochemical Energy Storage and Conversion) [Jung, Joey, Zhang, Lei, Zhang, Jiujun] on Amazon . *FREE* shipping on

Comparative study of intrinsically safe zinc-nickel batteries and lead
Therefore, it is highly required to develop next-generation electrochemical energy storage devices that can be alternatives with intrinsic safety for lead-acid batteries.

Past, present, and future of lead–acid batteries
of energy storage technologies. j Despite perceived competition between lead–acid and LIB tech-nologies based on energy density metrics that favor LIB in por-table

Electrochemical Energy Storage
Great energy consumption by the rapidly growing population has demanded the development of electrochemical energy storage devices with high power density, high energy density, and long

Global Electrochemical Energy Storage Market Size and Share 2031
Lead-acid batteries are an electrochemical energy storage method that has been used for a long time. Most lead-acid batteries consist of positive and negative electrodes are made of lead and

Lead Acid Battery
Lead-acid batteries are defined as the first rechargeable electrochemical battery storage technology, consisting of a cathode made of lead-dioxide and an anode of metallic lead,

Electrochemical Energy Storage
Electrochemical energy storage covers all types of secondary batteries. Batteries convert the chemical energy contained in its active materials into electric energy by an electrochemical

Electrochemical Energy Storage (EES)
Flow batteries can overcome the limitations of standard electrochemical accumulators (lead–acid or nickel–cadmium for instance) in which the electrochemical reactions create solid compounds that are stored directly

Lead batteries for utility energy storage: A review
Lead batteries are very well established both for automotive and industrial applications and have been successfully applied for utility energy storage but there are a range

Self-discharge in rechargeable electrochemical energy storage
This review focuses on the self-discharge process inherent in various rechargeable electrochemical energy storage devices including rechargeable batteries,

What is electrochemical energy storage and how it
The large-scale application of electrochemical energy storage technology needs to meet three basic requirements: high safety, high cost performance, and low pollution. At present, the most mature

(PDF) Multiphysics Engineered Next-Generation
This report explores advancements in lead-acid battery technology, focusing on innovations that enhance their application in electric vehicles (EVs) and energy storage systems. Despite the rise of
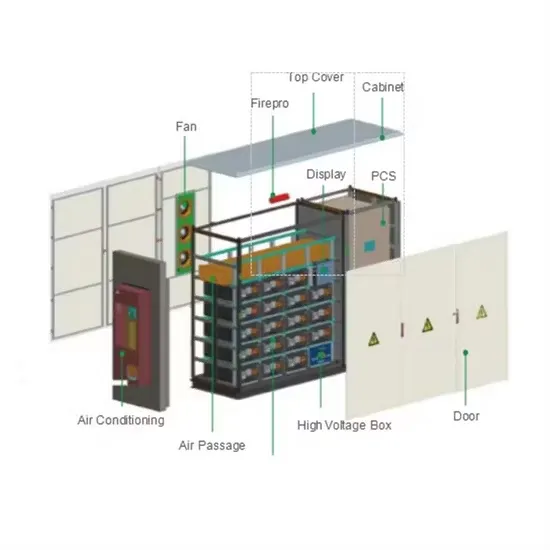
(PDF) LEAD-ACİD BATTERY
The lead-acid battery is the oldest and most widely used rechargeable electrochemical device in automobile, uninterrupted power supply (UPS), and backup systems for telecom and many other

lead-aCid battery
A lead-acid battery system is an energy storage system based on electrochemical charge/discharge reactions that occur between a positive electrode that contains lead dioxide

Advanced Lead–Acid Batteries and the Development of Grid-Scale Energy
This paper discusses new developments in lead-acid battery chemistry and the importance of the system approach for implementation of battery energy storage for renewable

Battery Storage
On its most basic level, a battery is a device consisting of one or more electrochemical cells that convert stored chemical energy into electrical energy. Each cell contains a positive terminal, or cathode, and a negative

Lead-Carbon Batteries toward Future Energy Storage: From
In this review, the possible design strategies for advanced maintenance-free lead-carbon batteries and new rechargeable battery configurations based on lead acid battery technology are

2.60 S2020 Lecture 11: Batteries and Energy Storage
Lithium Ion batteries The open circuit potential of a LiCoO2 battery is ~ 4.2 V. Specific energy is ~3-5X, specific power is 2X higher than lead-acid.~~~sfLCffbllllulsollo Table shows the

Electrochemical Energy Storage (EcES). Energy Storage in
Electrochemical Energy Storage (EcES). Energy Storage in Batteries Electrochemical energy storage (EcES), which includes all types of energy storage in batteries, is the most widespread

Lead-Carbon Batteries toward Future Energy
The lead acid battery has been a dominant device in large-scale energy storage systems since its invention in 1859. It has been the most successful commercialized aqueous electrochemical energy storage system ever

A comprehensive review on the techno-economic analysis of
This paper provides a comprehensive overview of the economic viability of various prominent electrochemical EST, including lithium-ion batteries, sodium-sulfur batteries,

Technology: Lead-Acid Battery
Summary of the storage process When discharging and charging lead-acid batteries, certain substances present in the battery (PbO2, Pb, SO4) are degraded while new ones are formed

Lead-acid batteries and lead–carbon hybrid systems: A review
Therefore, lead-carbon hybrid batteries and supercapacitor systems have been developed to enhance energy-power density and cycle life. This review article provides an
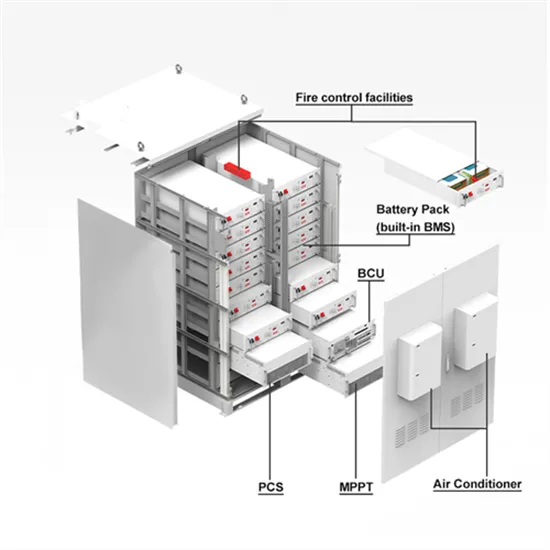
Lead‐Acid Battery
General Characteristics and Chemical/Electrochemical Processes in a Lead-Acid Battery Battery Components (Anode, Cathode, Separator, Endplates (Current Collector),
You might like this video
6 FAQs about [Lead-acid batteries in electrochemical energy storage]
Can lead-acid battery chemistry be used for energy storage?
Abstract: This paper discusses new developments in lead-acid battery chemistry and the importance of the system approach for implementation of battery energy storage for renewable energy and grid applications.
What is lead acid battery?
It has been the most successful commercialized aqueous electrochemical energy storage system ever since. In addition, this type of battery has witnessed the emergence and development of modern electricity-powered society. Nevertheless, lead acid batteries have technologically evolved since their invention.
What is a lead battery energy storage system?
A lead battery energy storage system was developed by Xtreme Power Inc. An energy storage system of ultrabatteries is installed at Lyon Station Pennsylvania for frequency-regulation applications (Fig. 14 d). This system has a total power capability of 36 MW with a 3 MW power that can be exchanged during input or output.
What is a lead-acid battery system?
1. Technical description A lead-acid battery system is an energy storage system based on electrochemical charge/discharge reactions that occur between a positive electrode that contains lead dioxide (PbO 2) and a negative electrode that contains spongy lead (Pb).
Why is electrochemical energy storage in batteries attractive?
Electrochemical energy storage in batteries is attractive because it is compact, easy to deploy, economical and provides virtually instant response both to input from the battery and output from the network to the battery.
What are the advantages of lead acid battery?
Lead acid battery Lead acid battery when compared to another electrochemical source has many advantages. It is low price and availability of lead, good reliability, high voltage of cell (2 V), high electrochemical effectivity, cycle life is from several hundreds to thousands of cycles.

